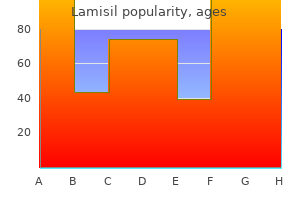
Lamisil

Stephen P. Glasser, MD
- Departments of Medicine and Epidemiology
- University of Alabama at Birmingham
- Birmingham, AL
For example fungal zygomycosis buy discount lamisil on line, with the combination of paclitaxel and cisplatin fungal growth buy 250 mg lamisil overnight delivery, an important regimen for ovarian cancer fungus gnats yellow sticky cards buy lamisil 250 mg mastercard, cisplatin reduces paclitaxel clearance if given first antifungal while pregnant lamisil 250 mg mastercard. For example fungi scientific definition buy 250 mg lamisil visa, capecitabine and lapatinib are used together in the treatment of metastatic breast cancer antifungal gel for sinuses buy lamisil 250mg with mastercard. Capecitabine is labeled to be taken twice daily with food, whereas lapatinib (which has a significant increase in exposure with food) is labeled to be taken once daily fasting. Besides drugs, many other extrinsic factors can influence exposure and response to anticancer therapies. What dosing recommendation should be made, if any, regarding administration of the product in relation to meals or meal types Small molecule non-cyotoxics that have been demonstrated to have an increase in exposure with food include erlotinib, lapatinib, nilotinib, pazopanib, vemurafenib, and abiraterone. The drug was labeled to be taken fasting at a dose of 750 mg daily because this is the dose that was used in the pivotal trial. Conclusions As illustrated earlier, there are examples from all classes of anticancer therapies that illustrate how an understanding of clinical pharmacology can enhance the therapeutic use of these agents. As the goal is to maximize efficacy while maintaining tolerability, the oncologist needs to understand the basic relationship between dose and exposure (pharmacokinetics), as well as exposure and response (pharmacodynamics) for the population as a whole. The oncologist also needs to understand how and why a certain patient might have a different response than an average patient, and why drug effects may vary over time (interpatient and intrapatient variability). As many new observations about the clinical pharmacology of anticancer therapies are made after drugs are marketed, a current review of the literature is always recommended as well. Some of the key concepts and content from the last edition were carried forward into the current version. Oral anticancer drugs: mechanisms of low bioavailability and strategies for improvement. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Proposed relationships between response and drug concentration in the intact animal and man. Implications of biochemical, cytokinetic, pharmacologic, and toxicologic relationships in the design of optimal therapeutic schedules. Axitinib in metastatic renal cell carcinoma: results of a pharmacokinetic and pharmacodynamic analysis. Imatinib plasma levels are correlated with clinical benefit in patients with unresectable/metastatic gastrointestinal stromal tumors. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. Relationships between pazopanib exposure and clinical safety and efficacy in patients with advanced renal cell carcinoma. Population pharmacokinetics and exposure-response analyses of trastuzumab in patients with advanced gastric or gastroesophageal junction cancer. Practical guidelines for therapeutic drug monitoring of anticancer tyrosine kinase inhibitors: focus on the pharmacokinetic targets. A general model for time-dissociated pharmacokinetic-pharmacodynamic relationship exemplified by paclitaxel myelosuppression. Pharmacokinetics and dosage reduction of cis-diammine(1,1-cyclobutanedicarboxylato)platinum in patients with impaired renal function. Basic concepts in population modeling, simulation, and model-based drug development-part 2: introduction to pharmacokinetic modeling methods. Patient characteristics associated with high-risk methotrexate concentrations and toxicity. Nonlinear pharmacokinetic models for 5-fluorouracil in man: intravenous and intraperitoneal routes. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. Effects of food on the clinical pharmacokinetics of anticancer agents: underlying mechanisms and implications for oral chemotherapy. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. A review of the pharmacokinetics and pharmacodynamics of combination carboplatin/paclitaxel. Phase I studies of sirolimus alone or in combination with pharmacokinetic modulators in advanced cancer patients. In addition, it is used for patients with nonmalignant diseases such as rheumatoid arthritis, psoriasis, autoimmune diseases, and graft versus host disease. Historical overview In the early 1940s, the combined observations that patients with acute leukemia often have serum folate deficiency and that the bone marrow megaloblasts of folate-deficient patients morphologically resemble leukemic blasts prompted some investigators to postulate that leukemia might be a result of a deficiency of this B vitamin. However, it rapidly became apparent that administration of folic acid to patients with leukemia was not only ineffective but often accelerated the course of the disease. Folate antagonists function in several ways: by competing with folates for uptake into cells, by inhibiting the formation of folate coenzymes, or by inhibiting one or more reactions that are mediated by folate coenzymes. This addition of up to seven or eight additional glutamate molecules serves to add mass and negative charge, markedly reducing efflux and increasing total intracellular accumulation at steady state. Absorption can be relatively poor and unpredictable,28,29 affected by food, nonabsorbable antibiotics, bile salts, and a shortened intestine transit time. Thus, it is suggested that the drug be taken on an empty stomach with clear liquids. The highest tissue-to-plasma concentrations found in humans are in the liver and kidney, followed by the gastrointestinal tract. Pharmacogenomics A growing body of data implicates inherited variation in genes for enzymes responsible for folate metabolism in interpatient variability in antifolate response or toxicity. A more detailed discussion of these data is beyond the scope of this chapter but has been the subject of comprehensive reviews. Some responses were noted with the 5000 mg/m2 dose regimen in patients who did not respond at lower doses. Early studies showed that twice-weekly therapy (20 mg/m2) was superior to continuous daily oral administration for treatment during remission. The response rate reported (approximately 30%) is similar to the 630 Chemotherapy response rate of the other most active single drug, cisplatin. This drug does have limited activity in small cell lung cancer and has been used in combination regimens to treat that disease. However, high-dose methotrexate has been one of the mainstays of treatment since its introduction in the 1970s. Genetic variants,71,134,135 subclinical folate deficiency, impaired renal function, a damaged marrow owing to previous radiation therapy, chemotherapy, or infection, and the use of trimethoprim-sulfamethoxazole for Pneumocystis carinii prophylaxis may predispose patients to hematologic (and gastrointestinal) toxicity. More severe gastrointestinal toxicity is manifest by diarrhea, which may progress to severe bloody diarrhea. When this occurs in association with neutropenia, patients are at high risk of typhlitis, sepsis, and death. Tissues that are self-renewing, with a higher S-phase fraction, are therefore at highest risk for damage by the folate antagonists. The effects on the marrow are dose related, but there is considerable Folate antagonists 631 has been shown to increase serum adenosine concentrations, which will decrease glomerular filtration rate. In cases of inadvertent overdosing (>100 mg intrathecally), fatalities have been reported. At its most severe, it presents with motor paralysis of the extremities, cranial nerve palsies, seizures, and even coma. While the pathogenesis of subacute antifolate-induced neurotoxicity is likely multifactorial, disruption to homocysteine homeostasis may play a pivotal role. The pathogenesis of delayed neurotoxicity may be a result of impairing folate-dependent methylation of components of the myelin sheath. Chest radiograph findings are nonspecific but may show patchy interstitial infiltrates. Histologic examinations show diffuse interstitial lymphocytic infiltrates, giant cells, and noncaseating granulomas. In some patients, a peripheral eosinophilia is observed, raising the possibility that this is an allergic pneumonitis. It manifests as an erythematous rash, characteristically noted on the neck and upper trunk. The rash may be pruritic and relatively insignificant and usually lasts for several days. Pleuritic and left-upper-quadrant pain, presumably attributable to splenic capsule inflammation, has been reported with a moderately high-dose regimen. This differential expression leads to selective sensitivity to folate antagonists relative to normal tissues. Other known polymorphisms selectively increase affinity for folates and increase the intracellular folate pool. The overall response rate among those with T-cell lymphomas was significantly higher than those with B-cell lymphoma, suggesting a possible selectivity of the T-cell malignancies. Supplementation with these vitamins in all subsequent regimens reduced toxicity and allowed delivery of a greater number of courses, increasing response rates. This study proved pemetrexed to be effective with an improvement in progression free and overall survival. Bart was a respected clinician, clinical researcher, teacher, and basic scientist with a legacy of many significant contributions to the (anti)folate field, including the identification of the folate-binding protein and description of its defining characteristics. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid (aminopterin). Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. Overexpression of the mitochondrial folate and glycine-serine pathway: a new determinant of methotrexate selectivity in tumors. Intensive oral methotrexate protects against lymphoid marrow relapse in childhood B-precursor acute lymphoblastic leukemia. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy-a Pediatric Oncology Group study. The enhanced cytotoxicity of combinations of 1-beta-D-arabinofuranosylcytosine and methotrexate. High-dose methotrexate for the treatment of primary cerebral lymphomas: analysis of survival and late neurologic toxicity in a retrospective series. Combination therapy with methotrexate and 5-fluorouracil: a prospective randomized clinical trial of order of administration. Phase 2B trial of aminopterin in multiagent therapy for children with newly diagnosed acute lymphoblastic leukemia. Carboxypeptidase G2 rescue in patients with methotrexate intoxication and renal failure. Dextromethorphan is effective in the treatment of subacute methotrexate neurotoxicity. Rescue of experimental intrathecal methotrexate overdose with carboxypeptidase-G2. A structurally altered human reduced folate carrier with increased folic acid transport mediates a novel mechanism of antifolate resistance. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. Inherent resistance of human squamous carcinoma cell lines to methotrexate as a result of decreased polyglutamylation of this drug. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Pemetrexed and gemcitabine as combination therapy for the treatment of Group3 medulloblastoma. In this articler, the various antimetabolites or analogs of uracil/thymine, cytidine/deoxycytidine, hypoxanthine/guanine, and adenosine that are currently used in the management of cancer are presented and reviewed with regard to metabolism, mechanism of action, clinical pharmacology, clinical activity, and toxicity. Uracil antimetabolites Over the years, there have been several uracil antimetabolites that have shown potential activity in the treatment of various malignancies. The development of pyrimidine and purine antimetabolite drugs has been based on the rationale that nucleic acids are critical to cell replication and, therefore, the pyrimidine and purine bases (and their nucleosides) that are the "building blocks" necessary to synthesize nucleic acids are themselves potential sites for designing drugs that could be effective in inhibiting nucleic acid synthesis, whether that be in bacteria, viruses, or tumor cells. The term "antimetabolite" refers to the ability of synthetic pyrimidines and purines to mimic the structure of the naturally occurring pyrimidine and purine bases (or nucleoside metabolites) and, therefore, to enter biochemical pathways similar to their natural counterparts. The resultant disruption in naturally occurring nucleic acid synthesis may occur in several ways. This has long been thought to be the primary mechanism by which fluoropyrimidine drugs work. It remains the major component of both adjuvant and advanced colorectal cancer regimens typically being administered with other drugs. Acute toxicities associated with intravenous bolus administration include myelosuppression, mucositis, and diarrhea. Clinical pharmacology Today, when FdUrd is used, it is mainly as a continuous hepatic arterial infusion through an implantable pump.
As a result fungus nail turning black order lamisil without a prescription, the method and perspective used to estimate costs of medical care must be carefully specified and can include payments made by the insurer (payer perspective) pyrithione zinc antifungal buy discount lamisil 250 mg line, out of pocket payments by the patient (patient perspective) vinegar for fungus gnats discount lamisil 250mg overnight delivery, total payments fungus jet fuel 250 mg lamisil free shipping, total charges antifungal for diaper rash purchase genuine lamisil, lost wages due to lost productivity natural antifungal yeast infection effective lamisil 250 mg, and the additional burden placed on family members or other caregivers (societal perspective). Many interventions may alter subsequent management strategies and outcomes, which may impact overall costs via these "indirect costs. Marginal costs indicate the additional cost of a change in treatment strategies or additional services and are often used to examine the incremental impact of changes in treatment strategies. Because of the complexity of calculating health care costs, and the potential variation in the cost for a given service, close attention should be paid to the details and underlying assumptions of any cost estimates. Bundled episodes of care Many payers no longer operate using an a-la-carte, fee for service system where each service or good is associated with a payment. Similarly, surgical procedures are often bundled, where a single reimbursement is expected to cover the surgery and all associated follow-up care, including complications within a predetermined timeframe. These systems are designed to incentivize providers and hospitals to provide efficient care and minimize complications. Cost-effectiveness analysis There are several types of cost analyses beyond simple summations that provide objective data with which patient, providers, payers, and policy makers can make informed decisions. Commonly analyses include cost-effectiveness analyses and modeling (described previously in this chapter). Alternative interventions are then compared in terms of cost per unit of effectiveness. Whether or not a specific intervention is considered "cost-effective" depends on the available resources and willingness to pay of the target population. A classic analysis of over 500 life-saving interventions suggested that the median cost per life year saved was approximately $42,000 per life year saved43 (in 1995 dollars), providing a rough benchmark of the relative willingness of the American public to pay. In contrast, global health initiatives, vaccinations, and the treatment of malaria are highly cost-effective at less than $100 per life year saved. A screening colonoscopy every 10 years beginning at age 50 costs roughly $11,000 per year of life saved. Such analyses provide a detailed and objective framework with which patients, providers, and policy makers can make rational tradeoffs in costs, care, and outcomes. Statistical aspects of the analysis of data from retrospective studies of disease. J Thorac Oncol: official publication of the International Association for the Study of Lung Cancer. Positron emission tomography and improved survival in patients with lung cancer: the Will Rogers phenomenon revisited. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. An update on randomized clinical trials in localized and locoregional prostate cancer. Selective referral to high-volume hospitals: estimating potentially avoidable deaths. End-of-life care for Medicare beneficiaries with cancer is highly intensive overall and varies widely. Oral contraceptive pills as primary prevention for ovarian cancer: a systematic review and meta-analysis. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. Use of patient-reported outcomes in randomized, double-blind, placebo-controlled clinical trials. Comparative effectiveness research under the Patient Protection and Affordable Care Act: can new bottles accommodate old wine In modern oncology drug development, determining which molecularly characterized tumors will benefit the most from a drug is a critical part of the development plan. In addition, another important personalized medicine approach is emerging whereby a therapeutic is specific to an individual patient. Examples of this individualized therapeutic approach include engineering of autologous immune cells with reinfusion into the patient or cancer vaccines that are specific to an individual tumor. A growing number of successful examples have demonstrated that the personalized medicine approach via biomarker selection can provide more benefit to patients and more rapid approval of drugs. The benefits of a personalized medicine strategy in oncology drug development are multiple and include an increased probability of overall success, smaller Phase 3 registration trials, and the potential for enhanced clinical benefits for patients. In a recent review, Falconi and colleagues examined the outcome of 676 Phase 1, 2, and 3 clinical trials of novel therapeutics for non-small cell lung cancer conducted from 1998 to 2012. However, when a biomarker was utilized for patient selection, the cumulative success rate increased by nearly sixfold to 62%. Thus, there is strong objective evidence for predictive biomarkers to enhance the probability of success in drug development. Using specific genetic and/or protein markers, a number of important drugs have been developed and approved in specific populations. This approach represents a paradigm shift that has proved so powerful that it is now a standard. Some successful examples of multiplex diagnostics include transcriptional profiling efforts. Using this approach, a gene expression pattern is identified for responders versus nonresponders. This profile is then prospectively applied to a validation set of samples to determine the predictive value, sensitivity, and specificity of the test. While analysis of cancer cells has formed the mainstay of personalized medicine, it is becoming increasingly clear that other components of a tumor, such as immune cells and stromal cells, are critical biologic mediators that can now be manipulated for therapeutic benefit. As new therapies target these microenvironment cells, new technologies for profiling are being developed. The complexity of measuring multiple markers in multiple cell types presents challenges in the development of novel immunotherapy. These include engineered autologous cells and individual tumor antigen approaches and are discussed in detail below. Thus, while personalized medicine has finally become a reality in oncology drug development, it is becoming a far more complex proposition as we address more complex mechanisms and biology. In this articler, we try to cover some of the key issues that are critical to understand in order to achieve further successes. Introduction the growing armamentarium of biomarker tools will allow a further expansion of the personalized drug development approach. It will also increase complexity across development and this will come with challenges. While precision medicine is now a reality, the successes have generally been where the companion diagnostic is the same as the target of the drug. As the science advances, efforts will need to be expanded in a few areas of personalized medicine to realize further gains. With few exceptions, most successful personalized medicine programs in oncology have measured a single gene or protein. As multiplexed approaches become mainstream with the analysis of thousands of datapoints, diagnostics using full panels will begin to emerge. This may require different clinical development strategies with Role of biomarkers and companion diagnostics in personalized medicine Types of biomarkers Biomarkers and their associated diagnostics are critical components of most personalized drug development efforts. All of these biomarker types contribute in critical ways to drug development, but we typically think of predictive biomarkers as being the driving force in personalized medicine. These are used to identify patients within a population who have a higher probability of responding to treatment than the "all-comer" population. The symbiotic development of molecular profiling technologies and the completion of the human genome sequence created new opportunities for the comprehensive discovery of markers to help predict drug efficacy or reduce risk of drug toxicity. In effect, this is the equivalent of two biomarkers for every gene in the human genome! This suggests that the term biomarker is being greatly overused as we simply do not have this many useful biomarkers to support all different research applications. The vast majority of these biomarkers are poorly characterized and cannot be used effectively in research or clinical applications without considerable efforts to develop analytically valid assays as well as correlative data to confirm their role as a biomarker of a biological process. Prognostic markers are developed to determine the likely course of disease in the absence of any specific pharmaceutical intervention and have been widely used in some oncology indications to predict the likelihood of disease recurrence after resection of the primary tumor. Predictive biomarker tests are used to predict response to a specific treatment and are discussed in detail below. Increasingly, biomarker strategies are being used to detect and follow emergent resistance to therapy and considerable success has been achieved for the class of tyrosine kinase inhibitors to predict resistance to therapy. Several important factors emerge upon close examination of the approved companion diagnostics in this table. Secondly, all of the approved companion diagnostics in oncology are for signal transduction inhibitors with driver mutations in the target or downstream pathways. Why is it that we have been successful in developing companion diagnostics for signal transduction inhibitors but not for other important oncology drug targets The answer is mainly that these driver mutations are the target (or closely related to the target) of the drugs being developed. Consequently, it is possible to quickly test the hypothesis that proliferation and metastasis of a tumor are driven by somatic abnormality detected in the drug target and that only those patients with such a somatic aberration will respond to inhibition of this target. This hypothesis can begin to be confirmed or refuted in clinical studies as soon as an efficacious dose is established in Phase 1 studies. In contrast, if samples have to be collected in Phase 2 for molecular profiling in order to develop a hypothesis, the development of a predictive marker becomes much more difficult. In this scenario, a stepwise approach must be taken which includes biomarker hypothesis generation, hypothesis testing and validation, and, finally, diagnostic development. One recent example of this complexity is seen with the development of immuno-oncology agents. However, early clinical data show that measuring the expression of the target checkpoints is quite complex as a predictive biomarker. Consequently, simply testing for the expression of the checkpoint target may be insufficient as a predictive marker for this new type of drug and we may have to develop new molecular profiles to predict response to these important immune-modulating drugs. Some have highly validated analytical performance and strong evidence of clinical utility, while other tests are much less well characterized. These panels are mostly used for research purposes and increasingly for screening cancer patients for enrollment into targeted clinical studies. Perhaps the best known of these tests is FoundationOne, provided by Foundation Medicine Inc. This information is analyzed and may be used by physicians to direct patients to appropriate clinical trials with targeted agents or, sometimes, off-label treatment with approved agents. Ideally, this begins at the same time a new therapeutic agent or target is identified. The predictive biomarker hypothesis outlines a strategy for identifying and testing assays that can prospectively predict drug sensitivity or resistance. As discussed above, in some programs, the predictive biomarker hypothesis is inherently defined by the drug target. For example, a program targeting a tumor resistance mutation might use the presence of that mutation as a predictive biomarker to select patients for early clinical trial evaluation to demonstrate rapid proof of concept. The specific characteristics of the proposed predictive biomarker test can influence its clinical validation. For example, a biomarker with a binary readout, such as the presence or absence of a mutation, is the simplest to evaluate in clinical trials because of the lack of ambiguity about what constitutes a positive test. In contrast, a continuous variable, such as intensity of immunohistochemical staining or the degree of gene amplification or copy number, is more complex because of the need to establish a threshold for test positivity in actual clinical studies. Even more complicated are multivariate tests, such as microarray signatures or composite parameters derived from a variety of different tests. In general, as these predictive biomarkers grow more sophisticated, the burden to conduct more highly powered clinical trials for clinical validation also increases. For example, the potency of a new therapeutic across a panel of molecularly characterized tumor cell lines can be explored and used to identify molecular signatures, or, ideally, individual gene alterations, that strongly associate with drug sensitivity or resistance. To date, complex gene signatures associated with drug sensitivity have not been useful 564 Individualized treatment as companion diagnostics; however, this approach might still be valuable as a tool for biomarker discovery. The prevalence of any newly identified candidate predictive biomarkers can be ascertained in archived tumor tissues to explore potential clinical relevance. Ultimately, the exploratory predictive biomarker hypothesis should yield a series of promising candidate assays for further evaluation in the clinic. Once a series of potential candidate predictive biomarkers are identified, the strength of the predictive biomarker hypothesis should be evaluated. The predictive potential of the associated candidate biomarkers can be explored in preclinical experiments conducted in relevant in vitro cell lines and in vivo tumor models. The presence of the predictive biomarker should strongly correlate with drug sensitivity over a series of tumor models. Confirmatory findings in patient-derived xenografts would further support the clinical relevance of the candidate predictive biomarker. The strength of the predictive biomarker hypothesis could substantially impact the choice of patient populations studied. If the predictive biomarker potential is highly compelling, then treatment in early trials might be restricted to biomarker-positive patients as a rapid proof-of-concept strategy. However, if the biomarker correlation is more tenuous, then treating both biomarker-positive and biomarker-negative patients early in clinical development is warranted. Ideal tests should be simple, robust, sensitive, and possess a rapid turnaround if used for patient screening. Analysis of circulating tumor cells, plasma proteins, or tests performed on formalin-fixed, paraffin-embedded tumor archival tissues are more advantageous than tests that require fresh tumor biopsies.
Despite considerable challenges including the absence of a precedent for the novel trial design fungus wrist watch generic 250mg lamisil mastercard, overall patient attrition antifungal soap for jock itch lamisil 250mg on line, and a diverse pharmacopeia fungi journals best 250mg lamisil, it was found that in 27% of 68 patients fungus gnats self watering pot lamisil 250mg mastercard, treatment selection guided by molecular profiling (gene expression microarray analysis) resulted in a longer progression-free survival than that during the most recent regimen on which the patient had progressed fungus gnats infestation buy lamisil 250mg lowest price. Streamlining this process will be critical to expansion of these tools beyond major academic research centers fungus eating animal example discount lamisil 250mg otc. Large-scale genomic characterization of tumors in the context of clinical trials has allowed identification of predictive biomarkers-recurrent genomic alterations that identify patients most likely to benefit from a particular drug. These provide tremendous clinical value by allowing patient stratification to the most relevant treatment option and have been rapidly commercialized. A broadening array of sequencing tests that may aid in diagnosis and treatment decisions is available to oncologists and their patients. Over 100 academic centers and 50 commercial laboratories have made such tests available. Although cost is still prohibitive for widespread use, expanded panel-, exome-, or whole genome-based sequencing will continue to transform screening and diagnostics in coming years. However, routine clinical use will not only require further cost reduction but also more comprehensive data supporting genomics-correlated clinical outcomes to illustrate the benefit of such testing and off-label drug use. Promising signs already include the addition of procedural terminology codes and a preliminary list of 21 sequencing tests included in the fee schedule of the Centers for Medicare and Medicaid Services. Finally, germline genomic analysis (whether part of hereditary cancer testing or conducted in tumor-matched normal tissue) entails the high likelihood of incidental secondary findings of putatively pathogenic genomic variants with unclear disease association and therefore presents a challenge. Widespread controversy exists over the balance between patient autonomy and the perception that patients knowledge of this information would result in physical or mental harm. Although not new, historical consideration of this concept has largely been confined to a process of linear clonal evolution driving the development of an increasingly malignant cancer phenotype. As a result, multiple related, but distinct clonal lineages may coexist within a single patient. The cornerstone of cancer evolution lies in the accumulation of genomic mutations, ultimately impacting cellular phenotype. The entire process from biopsy to treatment plan is designed to be performed in less than 5 weeks. Although the majority of acquired cancer mutations are likely to be passengers, a small proportion will impact critical cellular pathways and processes, thereby acting as disease drivers. However, the exact molecular mechanisms leading to increased mutagenesis in cancer are incompletely understood. Recent analysis, carried out on thousands of cancer genomes, identified at least 20 distinct mutational signatures pointing to diverse processes driving mutagenesis in various cancers, with most cancers showing evidence of more than one such process at play. Elucidating such novel mechanisms of mutagenesis will be important for facilitating development of better prevention and treatment strategies. The accumulation of mutations clearly provides the raw material (population diversity) for cancer evolution. However, this accumulation often contributes to the process of clonal evolution within temporal and spatial constrains. The order of mutational events may also depend on the cell type and state of differentiation. In fact, such spatial separation could lead to "parallel evolution" characterized by distinct mutations arising independently in individual clones, but targeting the same gene or the same pathway, as observed in a variety of cancers. Although clonal diversification is crucial, recent data indicates that simple outgrowth of the most aggressive clones does not Cancer genomics and evolution 107 fully account for the extent of clonal evolution in cancer. Indeed, similar to the principles of evolutionary biology in general, where no species evolves in isolation, cancers seem to evolve within an ecosystem of interactions not only with the host but also with other coexisting tumor clones. Here, the presence of an invasive clone enables invasion by another, otherwise poorly invasive clone. Understanding of the molecular spectrum and mechanisms of cancer evolution impacts our ability to treat cancer. Molecular heterogeneity and clonal evolution limit the benefits derived from targeted treatments. Pre-existing somatic mutations (even passenger events) or new mutations acquired during therapy can drive therapeutic resistance. As can be seen, linear evolution can result in heterogeneity if a subclone has failed to outcompete its predecessors. Cancer genomics and evolution in clinical practice: A case study in melanoma Background Malignant melanoma is the sixth most common cancer in the United States and is one of few that is increasing in incidence. Dramatic recent progress in melanoma precision medicine powerfully illustrates the potential for genomics to impact clinical cancer management. In this section, we will focus on this progress and its impact on melanoma treatment while clinical features will be discussed in detail in Chapter 111. Melanoma is a disease of transformed melanocytes and is clinically subdivided by anatomic location, histopathology, sun exposure, and progression (using tumor-node-metastasis staging). This categorization has implications for prognosis and treatment, although it is sometimes unable to predict outcome or treatment response. Further, until the discoveries of the past decade, metastatic melanoma was uniformly treated with cytotoxic chemotherapy to limited effect. Melanoma genomic landscapes the striking genetic complexity between subtypes and within individual tumor genomes revealed by the above analyses was soon confirmed at high resolution through a succession of genomic studies. Melanoma, the deadliest of skin cancers, is caused by the transformation of melanocytes (pigment-producing cells) that accumulate genetic alterations, leading to abnormal proliferation and dissemination. Clinically, melanoma lesions can be classified based on location and progression, ranging from benign nevi to metastatic melanoma. For the two samples with both a matched primary and metastatic sample, only the mutation information from the metastasis was included. Several small studies of uveal, acral, desmoplastic, and mucosal melanoma have identified recurrent mutations, but much still remains unknown. Clinical implications of melanoma genomics Although no definitive correlation with outcome has been identified based on the above-mentioned genomic subtyping or other molecular classifications in melanoma, these genomic subtypes have direct bearing on treatment of advanced metastatic melanoma (Table 6). Nevertheless, early phase studies have shown sufficient efficacy to encourage ongoing efforts at developing agents for these patients. Only a minority of patients on the registration studies for pembrolizumab and nivolumab had noncutaneous melanoma and ocular melanomas were excluded. Similarly, genomic testing can accelerate new drug development and target identification for the less common subtypes of melanoma and potentially lead to identification of resistance pathways to guide drug selection in individual patients. The challenge, then, is to use available genomic technologies in a real time clinical setting. In theory, one could identify the evolutionary mechanisms active in the individual patient to guide treatment selection. In addition to molecular diversity across melanoma subtypes and even between individual patients with the same disease subtype, a higher order of complexity is becoming apparent. As outlined in the previous section on cancer evolution, recent data indicates that development of melanoma cannot be fully explained by a linear model of accumulating mutations leading to an increasingly aggressive phenotype. Instead, an emerging picture points to cancer as a complex ecosystem driven by the process of branched clonal evolution. As a result, multiple related, but distinct clonal lineages may exist within a single patient,251 with some lineages being more likely to progress and metastasize. As summarized in this chapter, genomics has already made extraordinary contributions to our understanding of cancer biology and cancer medicine. Nevertheless, the enormous potential of cancer genomics has only just begun to be realized. We also thank Matthew Taila and Victoria Zismann for assistance in preparation and proofreading of this chapter as well as Jeffrey Watkins for assistance in creation of figures. Phenomenon by which extensive chromosomal rearrangements occur in a single event in one or a few chromosomes. A mutation that confers a selective growth advantage on the cell in which it occurs. Mutation in the germ cell lineage present in all cells of the body and transmitted to offspring. Loss of the normal, functional allele at a heterozygous locus via deletion or other mutational event. High-throughput sequencing methodologies based on simultaneous sequencing of large numbers of genes or entire genomes. A mutation that does not confer a selective growth advantage to the cell in which it occurs. Single nucleotide sequence difference occurring in germ lines of individuals across populations. A gene safeguarding cellular processes that, when inactivated, facilitates oncogenesis. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Le Beau, PhD Overview the malignant cells of most patients who have leukemia, lymphoma, or a solid tumor have acquired clonal chromosomal abnormalities, the identification of which can aid in establishing the correct diagnosis and prognosis and in the selection of therapy. The advent of high-throughput methods, such as next-generation sequencing, capable of surveying the entire genome or large panels of cancer-related genes, presents new options for a revolutionary change in the way we diagnose, characterize, and treat cancer. Genetic consequences of genomic rearrangements Recurring chromosomal translocations result in alterations of the genes located at the breakpoints and play an integral role in the process of malignant transformation. There are two general mechanisms by which chromosomal translocations result in altered gene function in a dominant manner. The first is deregulation of gene expression, characteristic of the translocations in lymphoid neoplasms that involve the immunoglobulin genes in B lineage tumors and the T cell receptor genes in T lineage tumors, that results in the inappropriate or constitutive expression of an oncogene. The second mechanism is the expression of a novel, fusion protein, resulting from the juxtaposition of coding sequences from two genes, typically located on different chromosomes. A number of human tumors, particularly solid tumors, result from homozygous, recessive mutations. More than one mutation is required for the pathogenesis of human tumors; thus, an important aspect of cancer biology is the elucidation of the spectrum of chromosomal and molecular mutations that cooperate in the pathways leading to disease. Where known, we describe the cooperating mutations associated with specific cytogenetic subsets of leukemia, lymphoma, or solid tumors. Introduction Cancer is a heterogeneous group of diseases caused by the stepwise accumulation of numerous genetic and epigenetic aberrations altering the function of genes that regulate genome stability, cell proliferation and differentiation, cell death, adhesion, angiogenesis, invasion, and metastasis in complex cell and tissue microenvironments. The analysis of metaphase chromosomes provided our first broad glimpse into the genetic anatomy of a malignant cell and identified many of the basic abnormalities that characterize cancer, such as deletions, translocations, and gene amplifications. Specific cytogenetic abnormalities have been identified that are very closely, and sometimes uniquely, associated with morphologically distinct subsets of leukemia, lymphoma, or solid tumors. In the hematologic malignancies, patients with favorable prognostic features benefit from standard therapies with a well-known spectra of toxicities, whereas those with less favorable clinical and cytogenetic characteristics may be better treated with more intensive or investigational therapies. The disappearance of an abnormal clone is an important indicator of complete remission following treatment, whereas the appearance of new abnormalities signals clonal evolution and, often, more aggressive behavior. Similarly, in solid tumors, the detection of a recurring cytogenetic abnormality or genetic change may inform the selection of targeted therapy or an investigational clinical trial. Given the rapid progress in genomic analysis, one can envision a new approach to patients with cancer based on molecular profiling of the malignant cells as well Holland-Frei Cancer Medicine, Ninth Edition. The observation of at least two cells with the same structural rearrangement, for example, translocations, deletions, or inversions, or gain of the same chromosome, or three cells each showing loss of the same chromosome, is considered evidence for the presence of an abnormal clone. An exception to this is a single cell characterized by a recurring structural abnormality, which likely represents the karyotype of the malignant cells. The major disadvantage is the inability to interrogate more than a few abnormalities. The main disadvantage of these assays is that the number of clinically relevant gene alterations per tumor has grown to the extent that high-throughput assays are becoming more efficient and cost-effective. Unlike karyotyping, they do not require dividing cells, an advantage for solid tumors. Assays are evolving rapidly, driven by advances in instrumentation, design, software, and decreasing cost. Generally, small amounts of material are required, and some tests are available even for fine needle aspirates. High tumor cellularity is preferred, although lower cellularity can be compensated for by greater sequencing depth. Compared to whole genome sequencing, they are cost-effective, provide greater sequencing depth, high sensitivity, the shortest turn-around-time, and decreased bioinformatics burden, as well as circumventing issues of incidental findings. The cost is lower than whole genome sequencing, but there is limited utility in detecting translocations. Most cells have two copies of the centromere of chromosome 17; however, polyploid cells have more copies. The number of reads that align to a region are compared to either normal tissue from the same patient, or a database of normal, diploid patient samples. Regions of amplification will have a higher number of reads as compared to normal. Regions with heterozygous or homozygous deletions will have relatively fewer reads than expected. As translocations involving two genes often have heterogenous breakpoints occurring in large regions of intronic sequence, capture probes have to be designed to span this intervening sequence. About 70% of the patients show additional abnormalities, commonly with +der(22)t(9;22),+21, or -7 (associated with a poorer outcome). Disease Chromosome abnormality Frequency Involved genes Consequence Acute lymphoblastic leukemia Precursor B t(12;21)(p13. The number in the parentheses refers to the frequency within the morphological or immunological subtype of the disease.
This should particularly be considered in light of the relatively low level of toxicity seen in the use of cancer vaccines fungus gnats running order 250mg lamisil amex. Acknowledgments the authors gratefully acknowledge the assistance of Debra Weingarten in the preparation of this chapter fungus gnats vector discount 250mg lamisil amex. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits fungus gnats jade plant discount lamisil online visa. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell-mediated cancer immunotherapy zeasorb-af antifungal powder uk cheap 250mg lamisil otc. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation antifungal for lips buy lamisil online from canada. Complete molecular remissions induced by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor against lymphoma fungus on plants generic 250 mg lamisil. Induction of immune responses in patients with B-cell lymphoma against the surface-immunoglobulin idiotype expressed by their tumors. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Peptide vaccination of patients with metastatic melanoma: improved clinical outcome in patients demonstrating effective immunization. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. Fusion cell vaccination of patients with metastatic breast and renal cancer induces immunological and clinical responses. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Determinant spreading and tumor responses after peptide-based cancer immunotherapy. The adoptive transfer of T cells can reconstitute or enhance resident immunity, which is in contrast to the apparent limitations associated with vaccine-based strategies in recipients whose endogenous immune system may fail to respond to immunogen. The adoptive transfer of T cells can enhance resident immunity, which is in contrast to the limitations associated with vaccine-based strategies in patients whose endogenous immune system may fail to respond to an immunogen. In this articler, we will describe the current status of cell-based immunotherapy for cancer. These tumor types may be targeted by an exogenous source of ex vivo expanded effector cells. In general terms, five forms of T cells have been manipulated for human application. The successful administration of polyclonal populations of donor-derived or autologous T cells is based in part on defining the precise cellular effectors and identifying Holland-Frei Cancer Medicine, Ninth Edition. Early studies showed promising results35 with remarkable objective response rates of about 50% in metastatic melanoma. Specifically, those patients who achieved complete remissions (approximately 1/5 of all patients treated) remained disease-free 3 years or greater after treatment. As a consequence though, the loss of a normal immune defense system led to increased rates of opportunistic infections. Non-myeloablative lymphodepletion regimens with cyclophosphamide and fludarabine achieved response rates reaching 51%. The desired population of autologous effector cells can then be sorted, activated, and expanded in vitro and infused into the patient to induce tumor regression and long-term immunoprotection. The very low frequency of T cells recognizing commonly expressed antigens limits the applicability of this approach. They not only provide effective treatment for patients but also supply a rich source of data for immunobiological discovery. Genetically engineered T cells With advancements in immunology and gene transfer, T-cell function has been enhanced by altering receptor specificity and signaling functions that control ability to recycle antigen-dependent effector functions. Despite early concerns about insertional mutagenesis, decades of experience with transduction of primary T cells have failed to show any examples of this theoretical complication. The most commonly used approaches are transposon/transposase systems such as Sleeping Beauty. This system leads to more efficient transduction and a greatly expanded number of T cells for subsequent adoptive transfer. Initial clinical efforts adoptively transferred autologous T cells that had been rendered specific for melanoma. This has been demonstrated in the mouse72,73 but has not led to clinical compromise in human trials. Methods to increase affinity are also not subject to thymic selection and tolerance mechanisms, which, when coupled with an incomplete understanding of their specificity, exposes the recipient to on target but off-tissue side effects. Most approaches currently rely on targeting nonmutated self-antigens, the most promising of which may be gene products from the cancer testis family of genes. Systemic administration of corticosteroids did not help to reverse the toxicities. While these events illustrate the potency that engineered autologous T cells can display, they highlight the urgent need for improved preclinical systems to uncover off-target adverse events before initiation of clinical trials. These clinical data underscore the need to understand the microenvironment in which genetically modified T cells operate as well as nongenetically modified T cells in their ability to participate in a fully competent T-cell activation event. The short duration of engraftment has made it more appealing to use as a bridge to transplant. On-target, but off-tumor, toxicities are a consequence of varying levels of expression of the targeted tumor-associated antigen in normal tissues. Redirected T cells can be highly potent and toxic to normal tissues that express low levels of the targeted antigen. This can be extremely detrimental as described in 2006 by the Erasmus University in Rotterdam. The severity of on-target, but off-tumor toxicities is dependent on whether the tissues expressing the targeted antigen are essential for survival, and if the injuries are manageable by other means. In vitro experiments will not always accurately predict these important data as the targeted antigen could be expressed only at certain stages of differentiation. Therefore, in vivo experiments in appropriate preclinical models should be considered. The type of T-cell subset that is engineered for adoptive immunotherapy is associated with various clinical outcomes. Animal studies have revealed that transfer of less differentiated T-cell populations has resulted in better in vivo expansion, persistence, and antitumor activity. With a shifting paradigm focusing on utilizing lesser differentiated T-cell subsets and preserving their memory characteristics, several avenues are being investigated to manufacture the best quality T-cell product for adoptive transfer. One main approach has been to modify the culture conditions by selection of choice cytokines, and their combination, from the common gamma chain cytokine receptor family. Full treatment included three infusions with two weeks between successive infusions. Unfortunately, Dendreon, the company which invented this innovative treatment, filed for bankruptcy in November 2014. Currently, hundreds of patients are being treated per year at individual academic centers. Dean Lee, Hiroki Torikai, Lenka Hurton, and George McNamara for their contributions to this chapter. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Cancer regression in patients after transfer of genetically engineered lymphocytes. Cardiovascular toxicity and titin cross-reactivity of affinityenhanced T cells in myeloma and melanoma. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. In vitro predictors of therapeutic response in melanoma patients receiving tumor-infiltrating lymphocytes and interleukin-2. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Allison, PhD Overview the basic principles that guide cancer immunology are immune surveillance, immune editing, and immune tolerance. Rapid increase in the knowledge of the mechanistic details of these basic principles has led to clinical success in the treatment of cancer. In this articler, we discuss the basic principles and recent advances in the field of basic and clinical immunotherapy that has given credence to the long-held belief that the immune system can be used to treat cancer. Further, we also focus on the role of combining different types of immunotherapies and other therapeutic modalities in the treatment of cancer. Immunosurveillance the idea that the immune system is capable of recognizing and responding to cancer is not novel. While it was established that chemically induced tumors were immunogenic in murine models, spontaneously arising tumors behaved differently and were not rejected in similar experimental systems. Moreover, profoundly immunosuppressed athymic mice did not have an increased frequency of tumors induced by a chemical carcinogen. Multiple strands of evidence would need to coalesce to rekindle enthusiasm for the therapeutic potential of immune-based strategies. Aline van Pel, Thierry Boon, and Pierre van der Bruggen demonstrated that specific immunity to spontaneous tumors could be induced by vaccinating mice with mutagenized tumor cells10 and that tumor-specific antigens could be recognized by human cytolytic T cells. The molecular definition of tumor antigens revolutionized the field of tumor immunology by legitimizing the mechanism by which the adaptive immune system discriminates between normal and neoplastic cells. The detection of tumor-specific responses in humans fueled speculation that these responses could be similarly manipulated to induce tumor eradication. They posited that driven by the selective pressure exerted by the immune system, the tumor cell population undergoes "immunoediting" becoming serially less immunogenic than the starting population, through a Darwinian environmental selection. Tumor cells from immunodeficient mice were more immunogenic than those from immunocompetent mice. Although sustaining the concept of immune surveillance, these data raised a potentially formidable obstacle to the delivery of clinically useful immunotherapies, suggesting that by the time a cancer becomes detectable it is already beyond the capabilities of the host immune system to eradicate it. Critically from the therapeutic standpoint, Holland-Frei Cancer Medicine, Ninth Edition. Data documenting the equilibrium process has remained relatively elusive and largely inferred from clinical observation. For example, the development of melanoma of donor origin in two recipients of renal allografts from the same donor who had been considered cured of melanoma treated 16 years previously. Immune suppression in these mice, however, resulted in the unmasking of dormant tumors, which then spread, ultimately killing the host. Furthermore, examination of stable dormant lesions revealed cancerous cells with similar morphological features to those in progressive lesions but with a lower proliferative index and increased apoptosis. The lesions were infiltrated with T cells suggesting a possible ongoing interaction with the host immune system. Engraftment of these cancerous cells into immunodeficient mice following a short period of ex vivo culture resulted in tumor growth. In contrast, transfer into immunocompetent recipients failed to induce tumor growth. Finally, tumor outgrowth occasionally occurred following a period of dormancy in immunocompetent animals, and in these cases, tumor cells were able to grow following transfer to immunocompetent mice, suggesting they had become less immunogenic. These data support the idea that tumor cells are unedited in equilibrium but become edited when they spontaneously escape immune control. Progression of cancer may not depend solely on intrinsic adaptions of the tumor cells to evade detection but rather on changes exerted on host immunity to permit tumor growth. These mechanisms are neither mutually exclusive nor entirely separable, as upregulation of the surface expression of immunoinhibitory ligands by tumor cells can potentially abrogate immune responses just as efficiently as downregulation of immunostimulatory elements. The apparent contradictions between this study and those supporting a more central importance for tumor-intrinsic editing may reflect differences in inherent tumor immunogenicity. More immunogenic tumors will, by definition, generate a more robust immune response.
250mg lamisil visa. Fungal Nail - Where have the Fungal Nails gone? #11 - [Throwback Thursday] (2019).


















